Table of Contents
STUDIES IN PHYSICAL CULTURE AND TOURISM
Vol. 11, No. 2, 2004
REVIEW ARTICLE
WŁODZIMIERZ MRÓWCZYŃSKI, PIOTR KRUTKI
University School of Physical Education, Poznań, Poland
Correspondence should be addressed to: Włodzimierz Mrówczyński, Department of Neurobiology, University School of Physical Education, Grunwaldzka 55, 60-352 Poznań, Poland, 
Table of Contents
Key words: spinal cord, ascending tracts, divergence, motor control.
Ascending tracts of the spinal cord constitute the essential elements of the somatosensory part of the nervous system. These pathways, originating from the lumbo-sacral enlargement, convey information from centers controlling hind limb muscles to higher spinal cord levels or to the structures located in the brain. They are commonly divided into short and long ascending propriospinal tracts, as well as long tracts ascending to supraspinal centers. The present knowledge enables us to suppose that many neurons of somatosensory tracts usually project to a single higher center. However, several groups of cells with branching axons that reach centers located at various levels of the nervous system have also been revealed. Such neuronal populations in the lumbar and sacral parts of the spinal cord have been well documented using either electrophysiological or morphological methods. Divergence of these ascending tracts suggests their many-sided contribution to the sensory system and enablesvarious spinal or supraspinal centers to process information of the same origin. From this point of view, some classifications of ascending spinal tracts appear to be imprecise. The aim of the present paper is to review and discuss most important questions related to axonal branching of the spinal cord neurons located at the lumbo-sacral level.
The ascending tracts are one of the essential elements forming the sensory neuronal network of the spinal cord. Application of the contemporary research methods, either anatomical (basing on axonal transport of specific enzymatic and fluorescent markers) or electrophysiological (e.g., recording antidromic action potentials from neurons), make a complex description of nearly each nervous tract possible. Locations of the cell bodies, synaptic inputs from various sources, peripheral or supraspinal, the axonal course in the spinal white matter as well as sites of terminations may be determined. Thus, each spinal ascending tract may be characterized by several properties. The most prominent features to describe are:
topographical distribution of individual neurons of origin of the investigated tract in the gray matter of the spinal cord (with respect to the cytoarchitectonic structure of the spinal cord segments [43]),
pattern of the afferent input, both monosynaptic and polysynaptic, from muscle, joint and cutaneous peripheral receptors,
convergence of information from the periphery, spinal interneurons and supraspinal descending pathways,
axonal course in funiculi of the spinal white matter and localization of terminal collaterals in various regions of the spinal cord, the brain stem or cerebellum,
axonal conduction velocity and its possible changes alongside the nerve fibers of the investigated tract,
location of the target neurons for each of the axonal projections,
functional significance of a given ascending tract, with respect to its contribution to the sensorimotor neural network.
Many ascending pathways described with regard to the above properties, form in general terms the second component (second order neuron – II, see Fig. 1 and 2) of the sensory ascending system. It is the essential part responsible for receiving sensory information from receptors and transmitting nervous signals to higher spinal levels or to the centers located in the brain.
The first element of this system (first order neuron – I, see Fig. 1 and 2), is responsible for reception and transformation of the stimulus, and initiation of the action potential. Its morphological basis is formed by pseudounipolar cells located in the spinal ganglia. The peripheral processes of these neurons differ structurally and take part in formation of individual receptors. Irrespective of the receptor location (internal or external) or structure (simple, e.g., free pain endings or composed, e.g., retina), its activation by a stimulus causes transformation of this information into a sequence of succeeding discharges (action potentials). The frequency of this neural code reflects properties of peripheral signals. This information ascends through peripheral nerve fibers to reach the spinal ganglia, and finally through dorsal roots of the spinal nerves is transmitted to the gray matter of the spinal cord. After entering the spinal cord, axonal ramifications of peripheral neurons form synaptic connections with the second order neurons.
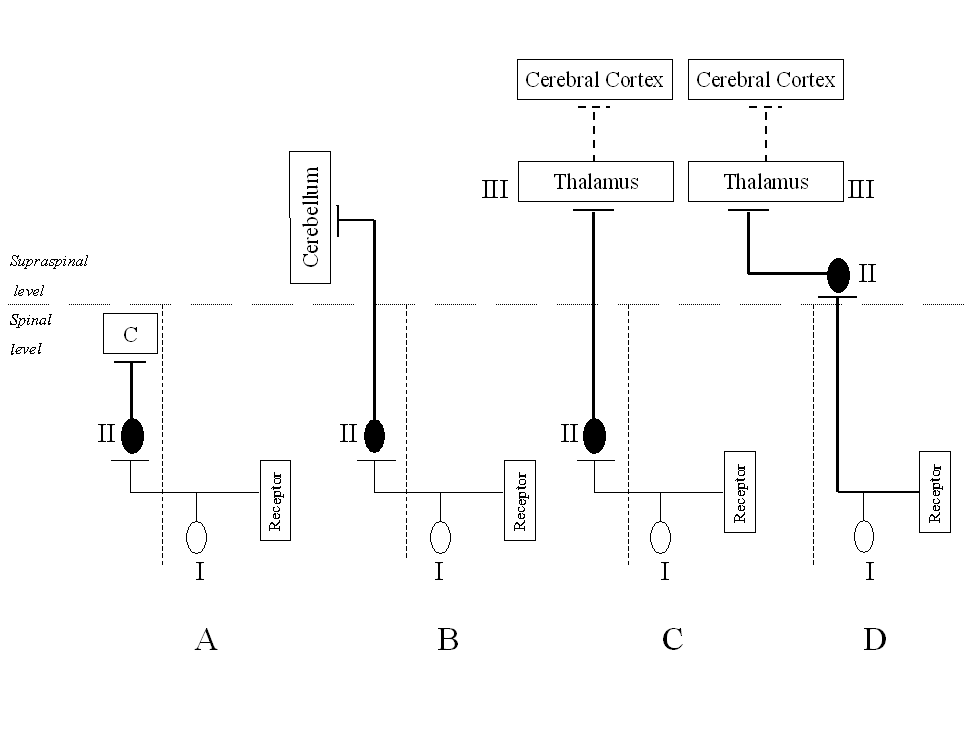
Figure 1. Simplified schemes of four main ascending tracts originating from the lumbo-sacral enlargement, responsible for transmission of sensory information from peripheral receptors A – long propriospinal tract projecting to the cervical enlargement (C6 segment); B – crossed spino-cerebellar tract; C – crossed spino-thalamic tract, as a part of long sensory pathway to the cerebral cortex from exteroceptors; D – spino-bulbar and bulbo-thalamic tracts, being components of the long sensory pathway to the cerebral cortex from proprioceptors. I – first order neurons (pseudounipolar cells of the spinal ganglia); II – second order neurons; III – third order neurons (for explanation, see text)
Spinal ascending tracts may be divided into two groups, with respect to the extent of their axons:
propriospinal neurons, with short axons forming connections between few neighbouring segments or with long intraspinal axonal projections, linking distant neuronal centers (usually between segments responsible for coordination of fore- and hindlimb activities, e.g., neurons of the lumbar origin with axons projecting to the cervical enlargement) (Fig. 1A),
neurons with axons forming long ascending tracts, projecting to neuronal centers located in the brain.
Second order neurons projecting beyond the spinal cord level may be further classified according to the direction of their axonal branching. Targets of these cells include subcortical nuclei of the brain stem, the thalamus (Fig. 1B) or the cerebellum (Fig. 1C). Some of these tracts form a part of longer sensory pathways between the spinal cord and the cerebral cortex. In such cases, neurons located in one of the brain stem nuclei or in the thalamus (i.e., the spino-thalamic, spino-reticular, spino-mesencephalic tracts) receive information from ascending tracts of the spinal cord and form third order neurons (III) of the ascending sensory system. The third order neuron delivers nervous signals to respective fields of the cerebral cortex. One of the spinal ascending tracts is usually described as a separate type of the three-neuronal sensory pathways to the cortex because it is formed by axons of the first order neuron (i.e., from spinal ganglia) ascending through the dorsal columns of the spinal cord directly to the medulla oblongata. This spino-bulbar tract in its major part consists of thick, group Ia sensory fibers, originating from muscle spindles, and makes terminal synaptic connections with neurons in the cuneate and gracile nuclei located in the bulbar region of the medulla. The second order neuron of this tract projects from the medulla to the contralateral thalamic nuclei (Fig. 1D).
Spinal ascending tracts as carriers of the nervous code have been investigated and described in detail. However, the above, simple classification of these pathways does not cover all possibilities of collateral axonal projections. It results from the fact that axons give ramifications on their course either in the spinal cord, or at the supraspinal level. One should also notice that collaterals are produced even by the axons of first order neuron ascending to the spinal cord, this way multiplying the number of ascending tract neurons that are supplied by the same kind of afferent information from peripheral receptors. This axonal divergence appears to be the common property of the spinal ascending tracts. In consequence, sensory information from receptors may be simultaneously processed by a number of the spinal and/or supraspinal neuronal centers allowing easier co-operation in response to the stimulus action (also during spinal reflexes). The result is the complex and adequate reaction of the organism to an operating factor. It is likely that the range of divergence has the key meaning for understanding the significance of the ascending part of the nervous system. The considerable contribution of neurons with axonal collaterals to the whole population of ascending tract cells may suggest a higher complexity of information transmission and processing than has been supposed previously. The most detailed research of this issue has been performed on mammals, mainly for tracts originating from lumbar and sacral segments of the spinal cord that conduct signals from centers controlling movements of hind limbs.
The majority of research on the neural network of the spinal cord has been performed on cats. A considerable number of neurons forming of the propriospinal system contain fibers running along almost the whole spinal cord. Most of them reach higher centers located in the thoracic or the cervical segments and terminate in Rexed's laminae V-VIII [3, 24, 28, 35, 36, 38, 44]. The neurons originating from these propriospinal connections are located in the lumbo-sacral enlargement either in the dorsal horn, the intermediate zone or the ventral horn of the gray matter. There are two clear-cut concentrations of these cells in the sacral and lumbar segments of the cord – the first one in laminae IV-VI, and the other in laminae VII and VIII [4, 5, 18]. Axons forming these pathways run mainly in the dorsal parts of lateral funiculi.
Long ascending propriospinal tracts, irrespective of the level of their projection sites, may be further distinguished with respect to their axonal course along the spinal cord: bilateral, ipsilateral or contralateral. The bilateral course of axons has been found most frequently among neurons ascending from the lowest segments of the spinal cord to cervical segments. This suggests that many propriospinal neurons convey sensory information to more then one spinal center. It is likely that both collaterals of these cells reach neurons in the forelimb motor centers, and this way they participate in motor coordination between fore and hind limbs during locomotion as well as voluntary movements. Similar conclusions have arisen from investigations on long propriospinal tracts in rats [15]. In this species the population of propriospinal neurons has been encountered in lumbar and thoracic segments (L3-Th11), in two groups, located in laminae V-VI and VII-VIII of the spinal gray matter. Pathways originating from these groups project ipsilaterally and bilaterally to the C7 segment of the cervical enlargement and terminate in lamina IX (see Fig. 2A).
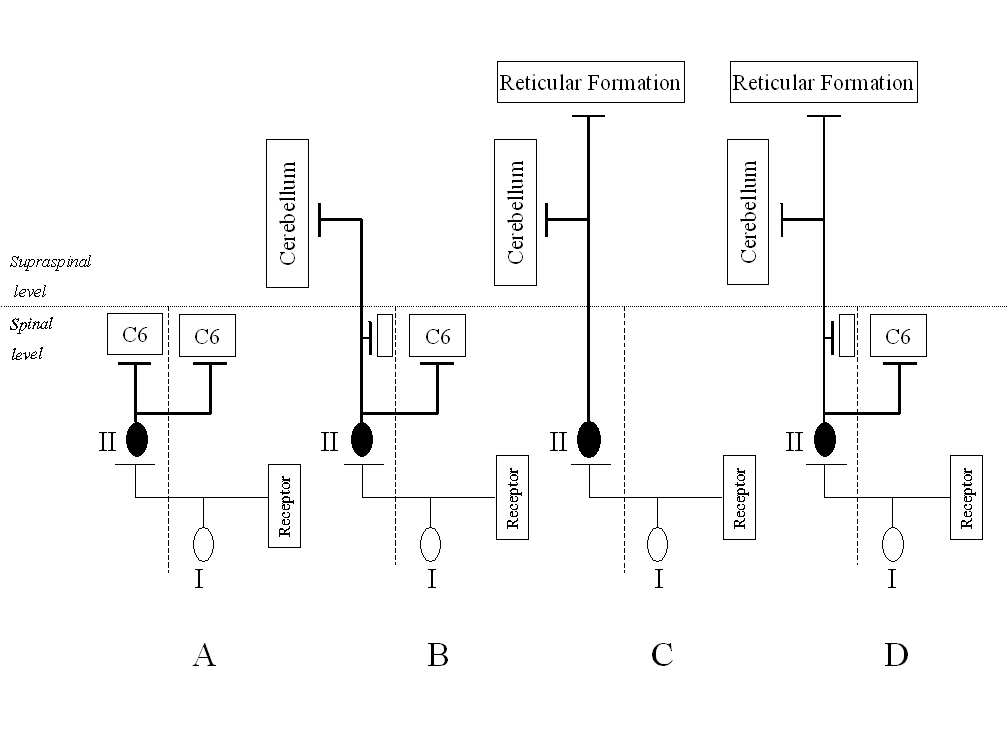
Figure 2. Examples of divergence in four groups of tracts ascending from the lumbosacral enlargement A – bilateral propriospinal projections to cervical segments of the spinal cord (C6); B – spino-cerebellar neuron with propriospinal collaterals to the sixth cervical segment (C6); C – dual ascending projections to the cerebellum and the reticular formation; D – triple projections to the cerebellum, the reticular formation and to the cervical spinal cord (C6). I – first order neuron (pseudounipolar cells of the spinal ganglia); II – second order neuron of sensory pathways from peripheral receptors
Numerous representations of neurons giving rise to spino-cerebellar tracts are located in sacral segments of the feline spinal cord. Three separated groups of cells, with regard to distribution of cell bodies, have been found [9, 11, 48]. They cover the area in the base of the dorsal horn and the intermediate zone of the gray matter and two regions in the ventral horn of S1-S2 segments, corresponding to the medial area of laminae VI and VII, lateral parts of laminae VII and IX, and the medial parts of laminae VII and VIII [29, 30]. Axons forming all these tracts ascend contralaterally, in the dorsal part of lateral funiculi and ach the cerebellar cortex in major part through the inferior cerebellar peduncle (restiform body) [23]. The projection areas in the cerebellum have also been precisely described with retrograde fluorescent double-labeling methods [49].
Electrophysiological studies performed later have revealed that the part of the spino-cerebellar population in the sacral segments include also neurons with dual, bilateral axonal projections. Moreover, a part of these cells located in laminae IV-VI and VII-VIII send axonal collaterals to both the cerebellum and the cervical (C6) segments of the spinal cord [39]. The fibers of these neurons also run in the dorsal part of lateral funiculi on the contralateral side. At least three basic types of the axonal course are to be distinguished. The most widespread are bilateral pathways to the cervical segments with additional long collaterals ascending to the cerebellum (Fig. 2B). Moreover, the neuronal subpopulation of neurons projecting to the cerebellum with collaterals ascending to the reticular formation of nuclei in the brain stem have been identified in the medial part of laminae VII and VIII of the gray matter. In this case all axons ascend unilaterally in the contralateral lateral funiculus [25]. Another subpopulation found at the same location, mainly in lamina VII of the sacral segments, and with similar contralateral axonal course, consists of cells with branching axons projecting dually to the cerebellum and the thalamic nuclei [16] (Fig. 2C). Furthermore, less numerous neurons with axons divided into several collaterals, projecting simultaneously to three different centers have been revealed in the feline sacral spinal cord. These pathways ascend bilaterally to the gray matter of the cervical segments of the spinal cord, as well as contralaterally to the reticular formation of the brain stem and to the cerebellum [12] (Fig. 2D). The location of these neurons in the gray matter of S1-S2 segments is fully consistent with the location of the previously described neurons of single and dual propriospinal and supraspinal projections.
In the lumbar and thoracic segments of the spinal cord, two main sensory tracts projecting from hind-limb centers to the cerebellum have been identified by means of neuroanatomical and electrophysiological methods and described in detail: the dorsal spino-cerebellar tract (DSCT) and the ventral spino-cerebellar tract (VSCT). Collateral connections of these tracts have been noticed; however, the divergence pattern resembling this, existing in the tracts originating from more caudal segments has not been established for any of them.
The DSCT originates from neurons of the thoracic nucleus extending for cats between the second and third thoracic segments (Th3-Th4) and the third and fourth lumbar segments (L3-L4). Cell bodies are located at the base of the dorsal horn, on the border with the intermediate gray matter. A minor part of DSCT cells are distributed also outside the thoracic nucleus at the level of L3-L4 segments [9]. The axons of this spino-cerebellar pathway ascend ipsilaterally in the dorsal part of the lateral funiculus and reach the cerebellum mainly through the inferior cerebellar peduncle. Studies on cats have indicated that a part of DSCT fibers give collaterals to the Z nucleus of the medulla oblongata. This center is commonly known as a relay nucleus for information transmitted originally from the muscle spindles and the Golgi tendon organs to the cortex of the brain [33].
The VSCT originates from the lumbar enlargement. Cell bodies originating from this tract are located mainly at the level of L3-L6 segments of the spinal cord, in the lateral part of the ventral horn and in the intermediate zone, with a minor part distributed also in the dorsal horn [10]. The VSCT fibers cross the midline and ascend in the ventral part of the ventral funiculus the contralateral side, reaching the cerebellum through the inferior peduncle. Some of VSCT axons give collateral branches to the lateral reticular nucleus of the brain stem [30].
Spino-thalamic tracts are usually described as second order neurons of tri-neuronal connection between peripheral receptors and the somatosensory cortex. Two main pathways are to be distinguished in mammals: the dorsal spino-thalamic tract (DSTT) and the ventral spino-thalamic tract (VSTT).
The DSTT is initiated from cells located predominantly in lamina I of the dorsal horn of the spinal gray matter. The axons of these neurons run in the dorsal part of the lateral funiculus on the contralateral side. About 25% of fibers comprised in this tract on their course at the brain stem level have originated from the lumbar enlargement. The DSTT axons ascend through the lateral funiculi of the spinal cord white matter, medulla oblongata, pons, midbrain and then as a lateral lemniscus reach the ventral nuclei of the thalamus. Information processed by DSTT neurons is mainly exteroceptive – from either pain or temperature receptors in the skin.
The VSTT originates from neurons located alongside the spinal cord, like DSTT neurons, but in the intermediate zone and the ventral horn of the gray matter, in regions corresponding to Rexed's laminae IV-V and VII-X. The axonal course of VSTT fibers is also similar to that of the DSTT; however, in the spinal cord it is limited to the ventral funiculi. Terminations of the axonal branches of this tract are found in the medial part of thalamic nuclei. The VSTT is responsible for transmitting the information received from touch and pressure receptors [20, 34, 46].
Studies on rats, cats and monkeys have indicated that the lumbo-sacral level of the spinal cord (as the region innervating for hindlimb muscles and skin areas) is one of the sites with a higher concentration of neurons giving rise to both tracts described above. Moreover, only minor interspecies differences have been pointed out. A significant number of DSTT and VSTT neurons located in the lumbar and sacral segments of catsare is distributed mainly in laminae I, lamina IV and V; and in laminae VII and VIII of the spinal cord [6, 20]. However, it has been revealed that their axons run at the spinal level both as crossed and uncrossed pathways in the lateral and ventral funiculi, and reach thalamic nuclei on both sides [6, 20, 34]. The fibers of the first group (corresponding to DSTT) project to the medial and lateral parts of the thalamus, while those of the second group (corresponding to VSTT) ascend mainly to intralaminar nuclei of the thalamus [7].
Experiments carried on rats have led to similar conclusions as in cats; neurons originated from the spino-thalamic tracts are numerous in the lumbo-sacral spinal cord and their cell bodies are distributed in laminae I, X, and from V to VIII, with both dorsal and ventral components of the spino-thalamic connections [22, 27]. Also in primates, two populations: DSTT and VSTT are present in the gray matter of the lumbo-sacral enlargement of the cord. Cell bodies are concentrated in laminae I, III and from lamina VII to X; axons run in lateral funiculi as well as in ventral funiculi [2].
No intraspinal branching has been reported for populations of the spino-thalamic tracts. However, collateral projections to the midbrain and the thalamus have been electrophysiologically proved in cats. Moreover, it has been evidenced that a significant part of the spino-reticular projections (15% of this population) that reach the reticular nuclei of the medulla oblongata through pathways ascending from the lumbo-sacral enlargement is formed by the axonal branches of the spino-thalamic neurons [14, 22, 47]. Huber et al. [16] have also evidenced that a part of neurons located in laminae VI and VII in sacral segments have dual projections to the thalamus and the cerebellum. Another kind of divergence has been found for some VSTT neurons that have branching axons terminating in the thalamus and in the periaqueductal gray of the brain stem [27].
Spino-reticular neurons are located in all segments of the spinal cord in rats, cats and primates [21, 22]; however, their representation is especially rich in both spinal enlargements [45]. The cell bodies are distributed mostly in Rexed's laminae VII and VIII, and a minor proportion of them is also dispersed in laminae I, V, and X. Axons of the spino-reticular tracts ascend mainly in ventral funiculi, although in some reports projections in lateral funiculi have also been described. The axonal course may be either crossed or uncrossed.
In the feline lumbo-sacral enlargement, the cell bodies of spino-reticular neurons are located in lamina V as well as in laminae VII and VIII [8, 32]. Their axons project in ventral and ventrolateral parts of the white matter, mainly to the pontomedullary reticular formation of the brain stem. The most caudal part of the spino-reticular system originates from the sacral segments (S1-S2). It has been electrophysiologically identified that cells are located predominantly in the medial part of lamina VIII [17], while the axonal course is predominantly contralateral and in some cases also ipsilateral, in the dorsal part of lateral funiculi.
Later investigations performed on cats have confirmed previous data related to the distribution of the spino-reticular neurons in the grey matter of the lumbar and sacral segments and additionally have revealed collateral projections of these neurons. Spino-reticular neurones from the medial parts of laminae IV-VI and VII-VIII have been found to project to the gigantocellular reticular nucleus (in the contralateral lateral funiculus) and to give collateral branches to neuronal centers located at higher levels of the spinal cord. A variable pattern of collateral branching, with ipsilateral, contralateral or bilateral projections to thoracic or cervical segments [16, 40] has been reported. The previous chapters discuss the divergence of spinal ascending tracts to the reticular formation, the cerebellum or the thalamus.
The inferior olivary complex is known as a center relaying information received from the upper cervical segments and from the lumbar enlargement to the cerebellum. Neurons originating from the spino-olivary tract from the lower levels of the spinal cord have been well investigated in the cat. They are located mainly in L3-L6 segments in laminae IV, V and VI [1, 37]. Other groups of cells are formed in the ventro-medial parts of the lamina VII in L5-S1 segments [31] and in the medial parts of laminae VII-VIII in sacral segments (S1-S2) [41]. The axons of the spino-olivary pathways are dispersed in all three funiculi, on the contralateral side of the cord. One must notice, however, that no collateral projections of the spino-olivary tract to other neuronal centers, neither in the spinal cord, nor in the brain, have been found thus far.
The reviewed studies have evidenced that the population of ascending tract neurons located in the lumbar and the sacral parts of the spinal cord is highly differentiated with respect to the distribution of cell bodies in the gray matter of various segments, as well as to the patterns of their spinal or supraspinal projections. Numerous studies on ascending tract neurons have concentrated on their functional contribution to motor actions or sensory feedback, by investigating afferent input from the periphery or from the brain. As a rule, the projections have been identified from one center only and the possibility of axonal divergence has not been checked. Thus, one may be convinced that neurons of this particular axonal projection to only one neuronal center are the main or unique group of cells located in a given part of the spinal cord. However, it should be stressed, that regions occupied by neurons of various ascending tracts overlap partly, with regard to respective segments of the spinal cord, to the distribution of cell bodies on transverse planes of the gray matter or to the course of axons in the ipsilateral/contralateral funiculi of the white matter. This observation leads to the suggestion that at least a proportion of the neurons in the lumbo-sacral enlargement, previously described as projecting to a single neuronal center, are in fact the same neurons with more than one axonal collateral. In several papers cited in previous chapters this point of view has been confirmed and authors have evidenced projections to more than one spinal or/and supraspinal centers.
Estimation of a relative proportion of branching neurons with reference to neurons of single projection is difficult to make with no doubt. Most studies indicate that as the number of identified centers of projection increases, the relative participation of these neurons in the total population becomes smaller. On the other hand, the extent of divergence cannot be measured precisely due to the electrophysiological techniques used in the majority of these studies that allow to record from statistically small groups of cells and enable the researcher to study at one time only a few, arbitrarily chosen projection sites in the spinal cord or in the brain. The knowledge of the exact proportion of ascending tract cells of multiple projections seems to be necessary for determining how much they participate in the neural network responsible for the transmission of sensory information and for reflex and feedback control of movements of the trunk hind limbs.
Many recent reports have revealed that the range of divergence may be even wider than previously suggested and concern ascending and descending spinal tracts as well. For example, subpopulations of neurons with branching axons terminating in more than one neuronal center have been found in the cervical enlargement of the cat’s spinal cord. Among them there are pathways bilaterally descending to the lower parts of the cord, with ascending collaterals to the lateral reticular nucleus [26], or neurons bi-directionally projecting both to the lumbo-sacral enlargement of the cord and to the reticular formation nuclei of the brain stem and the cerebellum [13]. Axonal branching has also been detected among pathways originating from the brain stem and descending to the spinal cord, e.g., vestibulo-spinal neurons projecting to cervical segments (C6) of the spinal cord that give collaterals the oculomotor nucleus [42].
The knowledge of axonal divergence of ascending tracts originating from the lumbo-sacral enlargement is still not complete. Reliable investigation of axonal divergence is limited because of two important factors. Firstly, research aims often comprise many aspects of morphology, pharmacological interactions and function of the ascending neural network, but exclude investigations of the divergence. Secondly, one cannot avoid damages to the nervous tissue made during preparation of too many parts of the nervous system, and there are still technical limitations of even advantageous electrophysiological methods. In practice, the last reason makes simultaneous investigations of collateral projections of a given tract to more than to three or four centers during one experiment impossible. Similar limitations concern morphological studies.
We can conclude that despite the above restrictions, a wide range of divergence of nerve fibers has been well documented for various animal species. Thus this phenomenon seems to be the universal property of spinal ascending tracts, whose axons bifurcate into various numbers of collaterals during its course. This way they distribute the same neural code of sensory information from spinal neuronal centers of the hind limbs to several higher centers. Moreover, many of these centers cooperate with each other during performed tasks. The complexity of such divergent actions of ascending pathways seems to be fundamental for proper processing of feedback information and for immediate corrections of movements controlled by subcortical areas. Finally, we should point out the relative and conventional character of the present classification of the spinal ascending tracts. After the divergence has been considered to be an additional criterion of differentiation of the described pathways, a proportion of neurons do not fit in the strict division into several single-projection groups. Therefore, a reasonable classification of ascending tracts originating from the lumbo-sacral cord should distinguish those with double or triple axonal projections from the long propriospinal, spino-cerebellar, spino-thalamic or spino-reticular tracts. The distinction of the diverging neurons will explain much better the complex process of transmission of sensory signals from the periphery and interrelations between numerous spinal cord and brain centers involved in sensorimotor control.
[1] Armstrong, D.M., Schild, R.F., Spino-olivary neurons in the lumbo-sacral cord of the cat, “Brain Research”, 1979, 168, pp. 176-179.
[2] Apkarian, A.V., Hodge, C.J., Primate spinothalamic pathways: II. The cells of origin of the dorsolateral and ventral spinothalamic pathways, “Journal of Comparative Neurology”, 1989, 288, pp. 474-492.
[3] Barilari, M.G., Kuypers, H.G.J.M., Propriospinal fibers interconnecting the spinal enlargements in the cat. “Brain Research”, 1969, 14, pp. 321-330.
[4] Brown, A. G., The spinocervical tract, “Progress in Neurobiology”, 1981, 17, pp. 59-96.
[5] Bryan, R.N., Trevino, D.L., Coulter, J.D., Willis, W.D., Location and somatotopic organization of the cells of origin of the spino-cervical tract, “Experimental Brain Research”, 1973, 17, pp. 177-189.
[6] Carstens, E., Trevino, D.L., Laminar origins of spinothlamic projections in the cat as determined by the retrograde transport of horseradish peroxidase, “Journal of Comparative Neurology”, 1978, 182, pp. 151-166.
[7] Craig, A.D., Kniffki, K.D., Spinothalamic lumbosacral lamina I cells responsive to skin and muscle stimulation in the cat, “Journal of Physiology”, 1985, 365, pp. 197-221.
[8] Fields, H.L., Wagner, G.M., Anderson, S.D., Some properties of spinal neurons projecting to the medial brain-stem reticular formation, “Experimental Neurology”, 1975, 47, pp. 118-134.
[9] Grant, G., Spinocerebellar connections in the cat with particular emphasis on their cellular origin, “Experimental Brain Research”, 1982, 6, pp. 466-476.
[10] Grant, G., Wiksten, B., Berkley, K.J., Aldskogius, H., The location of cerebellar projecting neurons within the lumbosacral spinal cord in the cat. An anatomical study with HRP and retrograde chromatolysis, “Journal of Comparative Neurology”, 1982, 204, pp. 336-348.
[11] Grottel, K., Huber, J., Kowalski, K., Functional properties of crossed spinocerebellar tract neurones with cell bodies in the S1 segment, “Neuroscience Research”, 1991, 11, pp. 286-291.
[12] Grottel, K., Krutki, P., Mrówczyński, W., Triple projections of neurons located in S1 and S2 segments of the cat spinal cord to the C6 segment, the cerebellum and the reticular formation, “Experimental Physiology", 1998, 83, pp. 737-746.
[13] Grottel, K., Krutki, P., Mrówczyński, W., Bidirectional neurons in the cervical enlargement of the cat spinal cord with axons descending to sacral segments and ascending to the cerebellum and the lateral reticular nucleus, “Experimental Physiology”, 1999, 84, pp. 1059-1071.
[14] Haber, L.H., Moore, B.D., Willis, W.D., Electrophysiological response properties of spinoreticular neurons in the monkey, “Journal of Comparative Neurology”, 1982, 207, pp. 75-84.
[15] Hiramatsu, K., Spinal afferents to lamina IX of the cervical enlargement in the rat studied by the retrograde transport of horseradish peroxidase, “Brain Research”, 1984, 292, pp. 375-377.
[16] Huber, J., Grottel, K., Celichowski, J., Dual projections of the ventromedial lamina VI and the medial lamina VII neurons in the second sacral spinal cord segment to the thalamus and the cerebellum in the cat, “Neuroscience Research”, 1994, 21, pp. 51-57.
[17] Huber, J., Grottel, K., Mrówczyński, W., Krutki, P., Spinoreticular neurones in the second sacral segment of the feline spinal cord, “Neuroscience Research”, 1999, 34, pp. 59-65.
[18] Jankowska, E., Lundberg, A., Roberts, W.J., Stuart, D., A long propriospinal system with direct effect on motoneurones and on interneurones in the cat lumbosacral cord, “Experimental Brain Research”, 1974, 21, pp. 169-194.
[19] Jones, M.W., Hodge Jr., C.J., Apkarian, A.V., Stevens, R.T., A dorsolateral spinothlamic pathway in cat, “Brain Research”, 1985, 335, pp. 188-193.
[20] Jones, M.W., Apkarian, A.V., Stevens, R.T., Hodge Jr., C.J., The spinothalamic tract: an examination of the cells of origin of the dorsolateral and ventral spinothalamic pathways in cats, “Journal of Comparative Neurology”, 1987, 260, pp. 349-361.
[21] Kevetter, G.A., Haber, L.H., Yezierski, R.P., Chung, J.M., Martin, R.F., Willis, W.D., Cells of origin of the spinoreticular tract in the monkey, “Journal of Comparative Neurology”, 1982, 207, pp. 61-74.
[22]Kevetter, G.A., Willis, W.D., Collaterals of spinothalamic cells in the rat, “Journal of Comparative Neurology”, 1983, 215, pp. 453-464.
[23] Kitamura, T., Yamada, J., Spinocerebellar tract neurons with axons passing through the inferior or superior cerebellar peduncles, “Brain Behaviour Evolution”, 1989, 34, pp. 133-142.
[24] Kostyuk, P.G., Maisky, V.A., Propriospinal projections in the lumbar spinal cord of the cat, “Brain Research”, 1972, 39, pp. 530-535.
[25] Krutki, P., Grottel, K., Mrówczyński, W., Divergence of lamina VII and VIII neurones of S1 and S2 segments of the cats spinal cord to the cerebellum and the reticular formation, “Acta Neurobiologiae Experimentalis”, 1999, 59, pp. 81-88.
[26] Krutki, P., Grottel, K., Mrówczyński, W., Branching neurons in the cervical spinal cord with axons that reach sacral segments and the lateral reticular nucleus, “Acta Neurobiologiae Experimentalis”, 1999, 59, pp. 279-285.
[27] Liu, R.P.C., Spinal neuronal collaterals to the intralaminar thalamic nuclei and periaqueductal gray, “Brain Research”, 1986, 365, pp. 145-150.
[28] Matsushita, M., Ueyama, T., Ventral motor nucleus of the cervical enlargement in some mammals; its specific afferents from the lower cord levels and cytoarchitecture, “Journal of Comparative Neurology”, 1973, 150, pp. 33-52.
[29] Matsushita, M., Hosoya, Y., Cells origin of the spinocerebellar tract in the rat, studied with the method of retrograde transport of horseradish peroxidase, “Brain Research”, 1979, 173, pp. 185-200.
[30] Matsushita, M., Hosoya, Y., Ikeda, M., Anatomical organization of the spinocerebellar system in the cat, as studied by retrograde transport of horseradish peroxidase, “Journal of Comparative Neurology”, 1979, 184, pp. 81-105.
[31] Matsushita, M., Yaginuma, H., Tanami, T., Somatotopic termination of the spino-olivary fibers in the cat, studied with the wheat germ agglutinin-horseradish peroxidase technique, “Experimental Brain Research”, 1992, 89, pp. 397-407.
[32] Maunz, R.A., Pitts, N.G., Peterson, B.W., Cat spinoreticular neurons: locations, responses and changes in responses during repetitive stimulation, “Brain Research”, 1978, 148, pp. 365-379.
[33] McIntyre, A.K., Proskie, U., Rawson, J.A., Corticifugal action on transmission of group I input from the hindlimb to the pericruciate cortex in the cat, “Journal of Physiology”, 1989, 416, pp. 19-30.
[34] Meyers, D.E.R., Snow, P.J., The morphology of physiologically identified deep spinothalmic tract cells in the lumbar spinal cord of the cat, “Journal of Physiology”, 1982, 329, pp. 373-388.
[35] Miller, S., Reitsma, D.J., Meché, F.G.A., Excitatory ascending proprospinal actions between lumbosacral and cervical segments in the cat, “Journal of Physiology”, 1971, 218, pp. 76-77.
[36] Miller, S., Reitsma, D.J., Meché, F.G.A., Functional organization of long ascending propriospinal pathways linking lumbo-sacral and cervical segments in the cat, “Brain Research”, 1973, 62, pp. 169-188.
[37] Molinari, H., Ascending somatosensory projections to the dorsal accessory olive: An anatomical study in cats, “Journal of Comparative Neurology”, 1984, 233, pp. 110-123.
[38] Mrówczyński, W., Lamina IV-VI neurones of the second sacral segment projecting to sixth cervical segment of the cats spinal cord, “Acta Neurobiologiae Experimentalis”, 1997, 57, pp. 189-195.
[39] Mrówczyński, W., Grottel, K., Krutki, P., Projections of laminae IV-VI neurones of the S2 segment of the spinal cord to the C6 segment and to the cerebellum in the cat, “Acta Neurobiologiae Experimentalis”, 1998, 58, pp. 103-111.
[40] Mrówczyński, W., Grottel, K., Krutki, P., Cervical and reticular projections of neurones located in S1 and S2 segments of the cat(s spinal cord, “Archives Italiennes de Biologie”, 1998, 136, pp. 237-245.
[41] Mrówczyński, W., Krutki, P., Electrophysiological investigation of spino-olivary projections originating from sacral segments of cat spinal cord, “Acta Neurobiologiae Experimentalis”, 2001, 61, pp. 319-324.
[42] Perlmutter, S.I., Iwamoto, Y., Baker, J.F., Peterson, B.W., Interdependens of spatial properties and projection patterns of medial vestibular tract neurons in the cat, “Journal of Neurophysiology”, 1998, 79, pp. 270-284.
[43] Rexed, B., A cytoarchitectonic atlas of the spinal cord in the cat, “Journal of Comparative Neurology”, 1954, 100, pp. 297-350.
[44] Rustioni, A., Kuypers, H.G.J.M., Holstege, G., Propriospinal projections from the ventral and lateral funiculi to the motoneurons in the lumbosacral cord of the cat, “Brain Research”, 1971, 34, pp. 255-275.
[45] Shokunbi, M.T., Hrycyshyn, A.W., Flumerfelt, B.A., Spinal projections to the lateral reticular nucleus in the rat: a retrograde labelling study using horseradish peroxidase, “Journal of Comparative Neurology”, 1985, 239, pp. 216-226.
[46] Trevino, D.L., Maunz, R.A., Bryan, R.N., Willis, W.D., Location of cells of origin of the spinothalamic tract in the lumbar enlargement of cat, “Experimental Neurology”, 1972, 34, pp. 64-77.
[47] Yezierski, R.P., Sorkin, L.S., Willis, W.D., Response properties of spinal neurons projecting to midbrain or midbrain-thalamus in the monkey, “Brain Research”, 1987, 437, pp. 165-170.
[48] Xu, Q., On the organization of axonal projections of spinocerebellar neurones from the lower part of the spinal cord. An experimental study in the cat. 1988, PhD dissertation, Stockholm.
[49] Xu, Q., Grant, G., Collateral projections of neurons from the lower part of the spinal cord to anterior and posterior cerebellar termination areas. A retrograde fluorescent double labeling study in the cat, “Experimental Brain Research”, 1988, 72, pp. 562-576.