Table of Contents
REVIEW ARTICLE
PIOTR GRONEK1, TADEUSZ RYCHLEWSKI1, RYSZARD SŁOMSKI2,3, KRYSTYNA STANKIEWICZ1, JOANNA LEHMANN1
1 Department of Physiology, Biochemistry and Hygiene, University School of Physical Education, Poznań, Poland
2 Department of Biochemistry and Biotechnology, Agricultural University Poznań
3 Institute of Human Genetics, Polish Academy of Sciences, Poznań
Correspondence should be addressed to: Department of Physiology, ul. Królowej Jadwigi 27/39, 61-871 Poznan, Poland, E-mail: 
Table of Contents
Key words: IGF-1, structure, function, physical training.
Insulin-like growth factors (IGFs) are mitogens for many cell types that play important roles in cell cycle progression, cell proliferation, and tumor progression. This paper discusses the insulin-like growth factor 1 (IGF-1). IGF1 is produced in numerous tissues and affects almost every cell. Major organs that synthesize IGF1 are the liver, heart, lung, placenta, kidney, pancreas, spleen, ovaries, large intestines, brain, small intestines, testes, bone and pituitary. In humans, approximately 10 milligrams of IGF1 are produced per day until the age of about 30 years whereby production decreases with aging. IGF1 is similar to insulin and possesses anabolic and cell growth effects. Insuline-like growth factor 1 is also important for its effect on development of diabetes and other chronic diseases. The major target tissues affected by IGF1 are muscle, cartilage, bone, liver, kidney, nerves, skin and lungs. The placenta is among the tissues that contain a high density of IGF-I receptors. IGF-1 is a 70 amino acidpolypeptide with a molecular mass of 7.6kD coded by the IGF-1 gene that contains 6 exons, 4 of which are alternatively spliced. It is involved in regulation of cell proliferation, differentiation and apoptosis. IGF1 stimulates skeletal muscle hypertrophy and a switch to glycolytic metabolism by activating the calcium calmodulin-dependent phosphatase calcineurin. Genetically determined low IGF-1 levels may lead to reduction in birth weight, length, and head circumference, and to persistent short stature and small head circumference in later life. It might be useful to gene therapy, which seems to be optimistic in the case of damaged muscle and dystrophies. In this brief review we address the advances that have been made in the studies on IGF-1.
Insulin-like growth factor 1 (IGF-1) belongs to a family of peptides that play important roles in mammalian growth and development mediating many of the growth-promoting effects of the growth hormone [64]. Its alternative name is somatomedin C. Investigations of Daughaday et al. [19] showed that the growth hormone (GH) did not directly stimulate the incorporation of sulfate into cartilage through a factor previously termed 'sulfation factor' and known as somatomedin. Recently, three main somatomedins have been recognized: somatomedin C (IGF-1), somatomedin A (IGF-2), and somatomedin B [80]. Human IGF1 is a 70-amino acid polypeptide cross-linked by 3 disulfide bridges, with a molecular mass of 7.6kD [77]. The liver is the primary source of circulating IGF-1 [90, 106]. The plasma concentration of IGF-1 reflects the secretion of the growth hormone (GH) by the pituitary gland.
The IGF-1 gene was mapped in chromosome 12 (Fig. 1). This gene contains 5 exons. Exons 1-4 encode the 195-amino acid precursor (IGF-1B); and exons 1, 2, 3, and 5 encode the 153-residue peptide (IGF1A) [80]. The structure of IGF-1 resembles the structure of IGF2. Smith et al. [92] did not confirm the 5-exon structure but they observed 6 exons, 4 of which were alternatively spliced depending on the tissue type and hormonal environment [92].
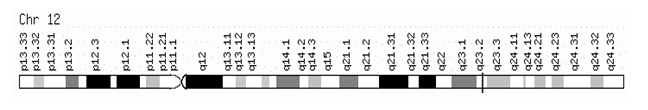
Figure 1. IGF-1 gene in genomic localization is found on cytogenetic band 12q23.2
The main functions of IGF-1 are presented in Table 1. IGFs are peptide hormones involved in regulation of cell proliferation, differentiation and apoptosis. IGFs are regulated by endocrine and paracrine processes. Their activity in tissue is determined by local production of IGFs and insulin-like growth factor binding proteins (IGFBPs). IGFs are predominantly bound to binding proteins (IGFBPs) [42, 106]. The insulin-like growth factor binding protein family has grown to six members, ranging in size from 216 to 289 amino acids. Four of these binding proteins are found in the serum. The synthesis of IGFBPs is regulated by IGFs and other growth factors [51]. IGFBP-3 is the predominant binding protein in the serum and it is also a marker of the growth hormone activity [46]. More than 90% of circulating IGF-1 is complexed with IGFBP-3. In fed or diet-restricted humans, administration of IGF-1 for several days appears to increase IGFBP-3 circulation [107].
Over the last few years we have witnessed a renewed interest in the chemistry and biology of the IGFBPs. The IGFBPs have their own intrinsic biological activities, independent of their ability to interact with IGF-I and IGF-II. IGFBPs directly regulate several cellular functions. Based on their primary structures, each IGFBP may be divided into three distinct domains of more or less equal size. The NH2- and COOH-terminal ends are highly conserved and contain 16–18 spatially conserved cysteine residues that form the various intra-domain disulfide bonds. Each IGFBP contains a GCGCCXXC motif and a CWCV sequence within their NH2- and COOH- end, respectively. Implicit in analysis of sequence homology has been the assumption that IGFBPs exhibit similar tertiary structures, including a “universal” IGF-binding domain.
Interaction between IGF-1 and its receptor IGF-1R is specific [109]. The signaling pathway mediated by Insulin-like Growth Factor 1 is shown in Figure 2 and a summary network of the IGF-1a precursor is shown in Figure 3.
The insulin-like growth factor receptor (IGF-1R) belongs to the family of transmembrane protein tyrosine kinases, which include the highly homologous insulin receptor and the insulin receptor related receptors. IGF1 stimulates skeletal muscle hypertrophy and a switch to glycolytic metabolism by activating the calcium calmodulin-dependent phosphatase calcineurin which was observed independently by Semsarian et al. [88] and Musaro et al. [62].
Semsarian et al. observed that skeletal muscle hypertrophy and regeneration were important adaptive responses to both physical activity and pathological stimuli [88].
Table 1. The recent investigations on IGF-1 function

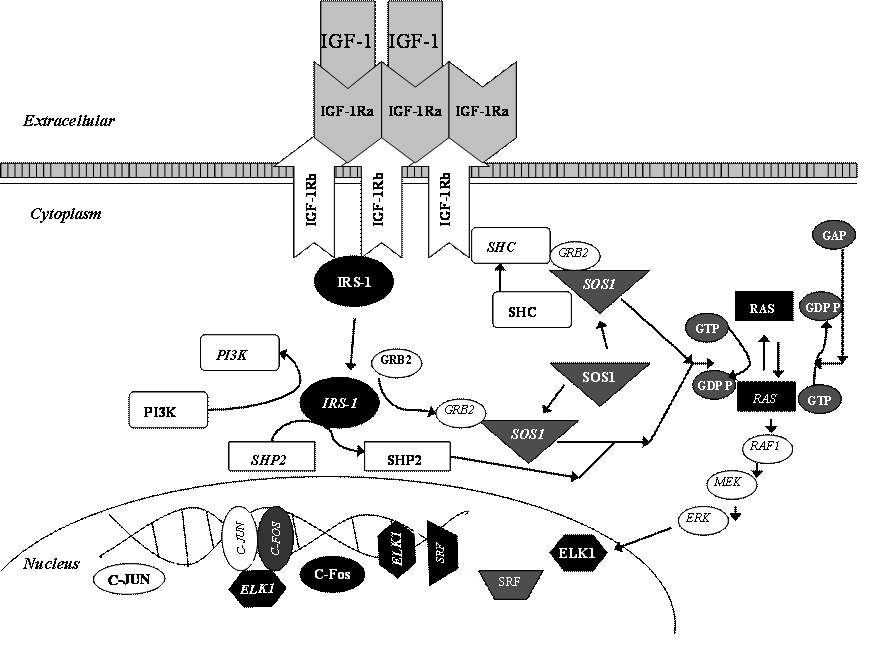
Figure 2. Signaling pathway mediated by Insulin-like Growth Factor 1
Skeletal cells synthesize insulin like growth factors (IGF) and six IGF binding proteins (IGFBP). IGFs are secreted by skeletal cells and their concentrations in the bone environment reach biologically significant levels. Failure to maintain these processes underlies the loss of skeletal muscle mass and strength that occurs with ageing and in myopathies. It has been known that stable expression of a gene encoding insulin-like growth factor 1 (IGF-1) in C2C12 skeletal muscle cells, or treatment of these cells with recombinant IGF-1 or with insulin and dexamethasone, results in hypertrophy of differentiated myotubes and a switch to glycolytic metabolism. When treating them using IGF-1 or insulin and dexamethasone it becomes clear that this mobilizes intracellular calcium, activates the Ca2+/calmodulin-dependent phosphatase calcineurin, and induces the nuclear translocation of the transcription factor NF-ATc1.
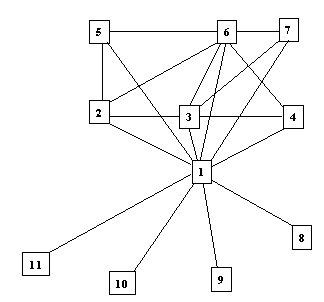
Figure 3. Summary network of IGF-1a precursor.1 – Insulin-like growth factor precursor 1a; 2 – Insulin-like growth factor receptor 1 precursor; 3 – Insulin-like growth factor binding protein 1 precursor; 4 – Insulin-like growth factor binding protein 3 precursor; 5 – Cation-independent mannose 6 phosphate receptor precursor; 6 – Insulin-like growth factor 2 precursor; 7 – Insulin-like growth factor 1B precursor; 8 – Ciliary neurotrophic factor; 9 – Myogenic factor; 10 – Growth hormone variant precursor; 11 – Interleukin-1 alpha precursor.
Once a plasmid encoding IGF-1 is injected into rat latissimus dorsi muscle it also activates calcineurin, mobilizing satellite cells and causing a switch to glycolytic metabolism. It is proposed that growth-factor-induced skeletal-muscle hypertrophy and changes in the myofibre phenotype, which are mediated by calcium mobilization, are critically regulated by the calcineurin/NF-ATc1 signalling pathway [88].
Expression of a dominant-negative calcineurin mutant also repressed myocyte differentiation and hypertrophy [62]. It was demonstrated that IGF-1 induced expression of transcription factor GATA2, which accumulates in a subset of myocyte nuclei, where it associates with calcineurin and a specific dephosphorylated isoform of NFATC1 [62].
There are numerous functions of the IGF-1 which have been described. An interesting study was conducted by Aleman et al. [1] which showed that IGF-1 levels were significantly associated with age-related reduction of certain cognitive functions, specifically the speed of information processing. Subjects with higher IGF-1 levels performed better on the Digit Symbol Substitution test and the Concept Shifting Task. The study supported the hypothesis that circulating IGF-1 might play a certain role in the age-related reduction of cognitive functions, specifically the speed of information processing.
Wodds et al. [105] identified homozygosity as responsible for partial deletion of the IGF-1 gene in a patient with severe prenatal and postnatal growth failure, sensorineural deafness, and mental retardation associated with IGF-1 deficiency. Another homozygous mutation in the polyadenylation signal of the IGF-1 gene in a patient with IGF-1 deficiency was identified by Bonapace et al. [9].
Insulin-like growth factor 1 and the receptor of the IGF-1 (IGF-1R) genes were considered to be candidates for insulin resistance, type II diabetes and low birth weight by Rasmussen et al. [75]. In genomic DNA from 82 Danish families with type II diabetes Rasmussen et al. [75] identified several silent and intronic polymorphisms.
Genetically determined low IGF-1 levels may lead to a reduction in birth weight, length, and head circumference, and to persistent short stature and small head circumference in later life [2]. Low IGF-1 levels were studied as a possibility of association between low birthweight and polymorphism of IGF-1. It seems that the consequence of the absence of the wildtype allele may be lower birthweight. An interesting question is whether the IGF-1 gene influences pre- or postnatal growth?
In the study conducted by Vaessen et al. [99] a correlation was observed between low birthweight and polymorphism in the IGF-1 gene that raises a risk of type 2 diabetes and myocardial infarction [99]. Individuals without the wildtype allele had a 215-gram lower birthweight than homozygous for the wildtype allele.
The activity of the hypothalamic–GH—IGF axis declines in midlife, leading to a relative GH and IGF-I deficiency. In conjunction with the many catabolic changes observed during aging, this fall in GH and IGF-I serum levels has been termed somatopause (Fig. 4).
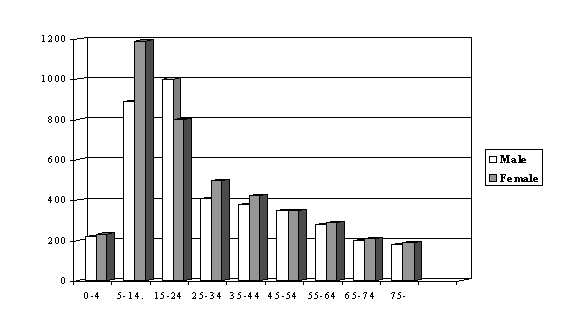
Figure 4. The activity of the hypothalamic–GH—IGF axis declines in midlife, leading to relative GH and IGF-I deficiency.IGF-1 levels is low at birth, increase during puberty and than start to decline during the 2nd-3rd decade of life. X axis: age span [years], Y axis: IGF-1 level [ng/ml]
Out side the muscle fibres residue are local satellite cells. The muscle-specific stem cells proliferate by normal cell division. As the next step some of the created cells fuse with the muscle fiber. Many factors are involved in this process including progrowth and antigrowth regulation factors. Satellite cells respond to IGF-1, and as a consequence there is a greater number of cell divisions; whereas a different growth regulating factor, myostatin, inhibits the process of their proliferation.
If the IGF-1 protein alone is injected to a cell it would dissipate within hours. But once a gene comes to a cell, it should keep functioning for the whole life duration of that cell, and muscle fibers are very long-lived. There are, therefore, many efforts to find a way to deliver the IGF-1 gene directly to muscle tissue [97].
The IGF-1 gene given to mice by the University of Pennsylvania researchers triggered additional production of insulin-like growth factor 1. Muscle strength was maintained and recovery from injury was as efficient as it was at a young age. In the experiment the strength and healing ability was seen throughout the lives of the treated mice. However, their life usually last a few years. It is not known how long the effects might be maintained in humans, or whether there could be as yet some unknown long-term side effects.
Many researchers use viruses as a delivery vector, because they are skilled at transporting genes to the cells. Our research group uses plasmids to carry genes into the cell. However, there are many problems in gene therapy, whether it is virus, adenovirus or plasmid. After injection of AAV-IGF-1 (IGF-1 packed into an adenovirus), one can observe that the muscle overall size and the rate at which they grow is 15-30% greater than normal [97]. When Rosenthal created mice genetically altered for overproduction of IGF-1, the animals developed normally except skeletal muscle which was 20-50% larger that in wild mice [62]. What is important from the cardiologic point of view IGF-1 levels were elevated only in muscles but not in blood. A high plasma level may be connected with cardiac problems and risk of cancer. When AAV-IGF-1 was injected into the murine leg muscle in animals after two-month weight-training, the injected animals gained circa twice as much strength in this muscle as in the uninjected legs in the same animal.
On training completion, the injected muscles lost strength much more slowly than the unenhanced muscle. Unfortunately this knowledge may be used in gene doping, and one can expect that sooner or later we might face some serious problems concerning detection of genetic doping including IGF-1.
Circulating levels of IGF-1 are regulated by insulin and the growth hormone. Malnutrition, fasting, liver disease and other severe diseases are associated with low IGF-1 concentration [16]. IGF-1 concentration decreases with aging [53]. Exercise is another important regulator of IGF-1 levels [60].
Several cross-sectional studies have found significant positive correlations between fitness level and circulating IGF-1 levels [24, 43, 70]. Such a correlation indicates that physical training leads to an increased activity of the IGF system. Higher levels of circulating IGF-1 and increased amplitude of spontaneous GH pulses were found in trained young males as compared with the untrained ones [43, 48, 70]. Studies on animals demonstrated that longer periods of training resulted in increased IGF-1 gene expression in the skeletal muscular tissue [108].
In contrast, several studies on exercise training have reported decreased circulating IGF-1 levels [24, 25, 79]. Eliakim et al. [25] hypothesized that the IGF-1 adaptation to prolonged training consisted of a two-phase response: the first one being the catabolic phase and the second one the anabolic state. Jahreis et al. (1991) found a decreased circulating IGF-1 level after intensive training programme in female gymnasts, in whom energy expenditure exceeded energy intake.
Eliakim et al. [24] suggested that the generally increased circulating IGF-1 levels in fitter subjects might be related to IGFBPs. Increased IGFBP-3 proteolysis was reported after an acute bout of exercise [87]. Changes in IGFBP-3 proteolysis are believed to represent a compensatory mechanism inducing the increase of free IGF-1 concentration by lowering IGFBP-3 ligand affinity and thereby modifying IGF-1 bioavailability by releasing IGF-1 from its 150-kDa complex, consisting of IGF-1, intact IGFBP-3, and an acid-labile subunit [74]. Rosendal et al. [79] speculated that IGFBP-1 and IGFBP-2 had a greater impact than IGFBP-3 on the levels of free and total IGF-1 during an 11-week of exercise program. In this study the decrease of IGF-1 levels was accompanied by upregulated levels of IGFBP-1 and IGFBP-2 despite an increased IGFBP-3 proteolysis in previously untrained subjects. The effect of regularly performed exercise on IGFBPs levels is conflicting. Rosendal et al. [79] suggested an existence of a threshold for relative physiological stress to be exceeded in order for IGFBP-3 proteolysis to occur.
Over the last several years the interest in IGF-1 has evolved from a focus on the levels of free IGF-1, to an interest in a variety of other biological effects. IGF-1 levels correlated with muscle mass in men but not in women in a cross-sectional sample in the New Mexico Aging Process Study. In men, but not in women, higher IGF-I levels protected against loss of lean body mass. Many questions remain to be answered in this field, also including the precise biochemistry of its interactions with IGFBPs, with extra-cellular matrix proteins, with putative receptors, and with intracellular targets; as well as concerning the three-dimensional structure. The potential roles played by the IGF-1 in human diseases (cancer) remain to be elucidated, and new discoveries are likely to lead to a number of novel therapeutic opportunities. Also gene therapy with IGF-1 seems to bring human beings an opportunity to understanding the muscle process better. The subject of therapeutic applications of the IGFs is potentially very broad. Some researchers predict potential benefits associated with the recombinant human IGF-1 (rhIGF-1) or the recombinant human IGF-1/IGFBP-3 complex. The potential availability of rhIGF-1 will open possibilities for therapy for a wide range of conditions and provide an opportunity to clarify physiological endocrine mechanisms in humans.
Aleman, A., Verhaar, H.J.J., De Haan, E.H.F., De Vries, W.R., Samson, M.M., Drent, M.L., Van Der Veen, E.A., Koppeschaar, H.P.F., Insulin-like growthfactor-I and cognitive function in healthy older men, “Journal of Clinical Endocrinology and Metabolism”, 1999, 84, pp. 471- 475.
Arends, N., Johnston, L., Hokken-Koelega, A., van Duijn, C., de Ridder, M., Savage, M., Clark, A., Polymorphism in the IGF-I gene: clinical relevance for short children born small for gestational age (SGA), “Journal of Clinical Endocrinology and Metabolism”, 2002, 87 (6), p. 2720.
Baffoni, C., Benso, A., Broglio, F., Tassone, F., Bellone, S., Corneli, G., Rovere, S., Origlia, C., Aimaretti, G. The nutritional status has no major role in the age-related reduction of IGF-I secretion, “Journal of Endocrinology Investigation”, 2002, 25 (10 Suppl), pp. 53-54.
Bankowski, E., Palka, J., Jaworski, S., An expression of IGF-binding proteins in normal and pre-eclamptic human umbilical cord serum and tissues “Molecular and Cellular Biochemistry”, 2002, 237 (1-2), pp. 111-117.
Barbieri, M., Ferrucci, L., Ragno, E., Corsi, A., Bandinelli, S., Bonafe, M., Olivieri, F., Giovagnetti, S., Franceschi, C., Guralnik, J.M., Paolisso, G., Chronic inflammation and the effect of IGF-I on muscle strength and power in older persons, “American Journal of Physiology and Endocrinology Metabolism”, 2003, 284 (3), pp. 481-487.
Bideci, A., Cinaz, P., Hasanoglu, A., Tumer, L., Leptin, insulin-like growth factor (IGF)-I and IGF binding protein-3 levels in children with constitutional delay of growth, “Journal of Pediatric Endocrinology and Metabolism”, 2002, 15 (1), pp. 41-46.
Bleumink, G.S., Rietveld, I., Janssen, J.A., van Rossum, E.F., Deckers, J.W., Hofman, A., Witteman, J.C., van Duijn, C.M., Stricker B,H., Insulin-like growth factor-I gene polymorphism and risk of heart failure (the Rotterdam Study), “American Journal of Cardiology”, 2004, 94 (3), pp. 384-386.
Bonafe, M., Barbieri, M., Marchegiani, F., Olivieri, F., Ragno, E., Giampieri, C., Mugianesi, E., Centurelli, M., Franceschi, C., Paolisso, G., Polymorphic variants of insulin-like growth factor I (IGF-I) receptor and phosphoinositide 3-kinase genes affect IGF-I plasma levels and human longevity: cues for an evolutionarily conserved mechanism of life span control, “Journal of Clinical Endocrinology and Metabolism”, 2003, 88 (7), pp. 3299-3304.
Bonapace, G., Concolino, D., Formicola, S., Strisciuglio, P., A novel mutation in a patient with insulin-like growth factor 1 (IGF1) deficiency, (Letter)“Journal of Medicine Genetics”, 2003,40, pp. 913-917.
Boyne, M.S., Thame, M., Bennett, F.I., Osmond, C., Miell, JP., Forrester, T.E., The relationship among circulating insulin-like growth factor (IGF)-I, IGF-binding proteins-1 and -2, and birth anthropometry: a prospective study, “Journal of Clinical Endocrinology and Metabolism”, 2003, 88 (4), pp. 1687-1691.
Bubley, G.J., Balk, S.P., Regan, M.M., Duggan, S., Morrissey, M.E., Dewolf, W.C., Salgami, E., Mantzoros, C., Serum levels of insulin-like growth factor-1 and insulin-like growth factor-1 binding proteins after radical prostatectomy, “Journal of Urology”, 2002, 168(5), pp. 2249-2252.
Bustin, S.A., Dorudi, S., Phillips, S.M., Feakins, R.M., Jenkins, P.J., Local expression of insulin-like growth factor-I affects angiogenesis in colorectal cancer, “Tumour Biology”, 2002, 23 (3), pp. 130-138.
Cappola, A.R., Xue, Q.L., Ferrucci, L., Guralnik, J.M., Volpato, S., Fried, L.P., Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women, “Journal of Clinical Endocrinology and Metabolism”, 2003, 88 (5), pp. 2019-2025.
Chan, JM., Stampfer, M.J., Ma, J., Gann, P., Gaziano, J.M., Pollak, M., Giovannucci, E., Insulin-like growth factor-I (IGF-I) and IGF binding protein-3 as predictors of advanced-stage prostate cancer, “Journal of the National Cancer Institute”, 2002, 94 (14) pp. 1099-1106.
Chlebna-Sokol, D., Rusinska, A., Serum insulin/like growth factor I, bone mineral density and biochemical markers of bone metabolism in children with idiopathic osteoporosis, “Endocrine Regulations”, 2001, 35 (4), pp. 201-208.
Clemmons, D.R., Van Wyk, J.J., Factors controlling blood concentrationof somatomedin-C, “Clinics in Endocrinology and Metabolism”, 1984, 13, pp. 113-143.
Conti, E., Pitocco, D., Capoluongo, E., Zuppi, C., Ghirlanda, G., Crea, F., Andreotti, F., IGF-1 and macrovascular complications of diabetes: alternative interpretations of recently published data, “Diabetes Care”, 2003, 26 (5), pp. 1653-1654
Dall'Aglio, E., Salimbeni, I., Rocci, A., Mazzoni, S., Corradi, F., Cattadori, E., Visioli, S., Banchini, A., Valenti, G., Ceda, G.P., Modifications of blood pressure and IGF-I levels after weight loss in obesity, “Journal of Endocrinology Investigation”, 2002, 25 (10 Suppl), pp. 107-109.
Daughaday, W.H., Hall, K., Raben, M.S., Salmon, WD Jr, van den Brande, J.L., van Wyk, J.J., Somatomedin: proposed designation for sulphation factor.“Nature”, 1972, 235 (5333), p. 107.
Davila, N., Shea, B.T., Omoto, K., Mercado, M., Misawa, S., Baumann, G., Growth hormone binding protein, insulin-like growth factor-I and shortstature in two pygmy populations from the Philippines, “Journal of Pediatric Endocrinology and Metabolism”, 2002, 5 (3), pp. 269-276.
de Mello Coelho, V., Villa-Verde, D.M., Farias-de-Oliveira, D.A., de Brito, J.M., Dardenne, M., Savino, W., Functional insulin-like growth factor-1/insulin-like growth factor-1 receptor-mediated circuit in human and murine thymic epithelial cells, “Neuroendocrinology”, 2002, 75 (2), pp. 139-150.
Decraene, D., Agostinis, P., Bouillon, R., Degreef, H, Garmyn, M. Insulin-like growth factor-1-mediated AKT activation postpones the onset of ultraviolet B-induced apoptosis, providing more time for cyclobutane thymine dimer removal in primary human keratinocytes. “Journal of Biological Chemistry” 2002, 277 (36), pp. 32587-325879.
Digicaylioglu, M., Garden, G., Timberlake, S., Fletcher, L., Lipton, S.A., Acute neuroprotective synergy of erythropoietin and insulin-like growth factor I, “Proceedings of National Academy of Sciences USA”, 2004, 01 (26), pp. 9855-9860.
Eliakim, A., Brasel, J.A., Mohan, S., Barstow, T.J., Berman, N., Cooper, D.M., Physical fitness, endurance training, and the growth-hormone-insulin-likegrowth factor I system in adolescent females, “Journal of Clinical Endocrinology and Metabolism”, 1996, 81, pp. 3986-3992.
Eliakim, A., Brasel, J.A., Mohan, S., Wong, W.L., Cooper, D.M., Increased physical activity and the growth hormone-IGF-1 axis in adolescentmales, “American Journal of Physiology Regulatory. Integrative and Comparative Physiology”, 1998, 275, R308-R314.
Figueroa, A., Going, S.B., Milliken, L.A., Blew, R., Sharp, S., Lohman, T.G., Body composition modulates the effects of hormone replacement therapy on growth hormone and insulin-like growth factor-I levels in postmenopausal women, “Gynecologic and Obstetric Investigation”, 2002, 54 (4), pp. 201-206.
Fischer-Posovszky, P., Tornqvist, H., Debatin, K.M., Wabitsch, M., Inhibition of death-receptor mediated apoptosis in human adipocytes by the insulin-like growth factor I (IGF-I)/IGF-I receptor autocrine circuit, “Endocrinology”, 2004, 145 (4), pp. 1849-1859.
Foulstone, E.J., Savage, P.B., Crown, A.L., Holly, J.M., Stewart, C.E., Adaptations of the IGF system during malignancy: human skeletal muscle versus the systemic environment, “Hormone and Metabolic Research”, 2003, 35 (11-12), pp. 667-674.
Frederick, T.J., Wood, T.L., IGF-I and FGF-2 coordinately enhance cyclin D1 and cyclin E-cdk2 association and activity to promote G1 progression in oligodendrocyte progenitor cells, “Molecular and Cellular Neuroscience”, 2004, 25 (3), pp. 480-492.
Fu, X., Lu, Y., Lu, K., Liao, J., Relation between insulin-like growth factor-1 and insulin resistance in patients with acute stroke, “Zhonghua Yi Xue Za Zhi”, 2002, 82 (6), pp. 410-412.
Gamper, N., Fillon, S., Huber, S.M., Feng, Y., Kobayashi, T., Cohen, P., Lang, F., IGF-1 up-regulates K+ channels via PI3-kinase, PDK1 and SGK1, “Pflugers Archive”, 2002, 443 (4), pp. 625-634.
Gonzalez, E., Messi, M.L., Zheng, Z., Delbono, O., Insulin-like growth factor-1 prevents age-related decrease in specific force and intracellular Ca2+ in single intact muscle fibres from transgenic mice, “Journal of Physiology”, 2003, 552 (3), pp. 833-844.
Gordon, P.V., Paxton, J.B., Herman, A.C., Carlisle, E.M., Fox, N.S., Igf-I accelerates ileal epithelial cell migration in culture and newborn mice and may be a mediator of steroid-induced maturation, “Pediatry Research”, 2004, 55 (1), pp. 34-41.
Gruden, G., Araf, S., Zonca, S., Burt, D., Thomas, S., Gnudi, L., Viberti, G., IGF-I induces vascular endothelial growth factor in human mesangial cells via a Src-dependent mechanism, “Kidney International”, 2003, 63 (4), pp. 1249-255.
Guo, Z.Y., Shen, L., Feng, Y.M., The different energetic state of the intra A-chain/domain disulfide of insulin and insulin-like growth factor 1 is mainly controlled by their B-chain/domain, “Biochemistry”, 2002, 41 (34), pp. 10585-10592.
Gustafsson, H., Tamm, C., Forsby, A., Signalling pathways for insulin-like growth factor type 1-mediated expression of uncoupling protein 3, “Journal of Neurochemistry”, 2004, 88 (2), pp. 462-468.
Hameed, M., Orrell, R.W., Cobbold, M., Goldspink, G., Harridge, S.D., Expression of IGF-I splice variants in young and old human skeletal muscle after high resistance exercise, “Journal of Physiology”, 2003, 547 (1), pp. 247-254.
Hernandez-Valencia, M., Zarate, A., Ochoa, R., Fonseca, M.E., Amato, D., De Jesus Ortiz, M., Insulin-like growth factor I, epidermal growth factor and transforming growth factor beta expression and their association with intrauterine fetal growth retardation, such as development during human pregnancy, “Diabetes Obesity and Metabolism”, 2001, 3 (6), pp. 457-462.
Humbert, S., Bryson, E.A., Cordelieres, F.P., Connors, N.C., Datta, S.R., Finkbeiner, S., Greenberg, M.E., Saudou, F., The IGF-1/Akt pathway is neuroprotective in Huntington's disease and involves Huntingtin phosphorylation by Akt “Developmental Cell”, 2002, 2 (6), pp. 831-837.
Iglesias, P., Bayon, C., Mendez, J., Gancedo, P.G., Grande, C., Diez, J.J., Serum insulin-like growth factor type 1, insulin-like growth factor-binding protein-1, and insulin-like growth factor-binding protein-3 concentrations in patients with thyroid dysfunction, “Thyroid”, 2001, 11 (11), pp. 1043-1048.
Jahreis, G., Kauf, E., Frohner, G., Schmidt, M.E., Influence of intensive exercise on insulin-like growth factor 1, thyroid and steroid hormones in femalegymnasts, “Growth Regulation”, 1991, 1, pp. 95-99.
Jones, J.I., Clemmons, D.R., Insulin-like growth factors and their bindingproteins: biological actions, “Endocrinology Review”, 1995, 16, pp. 3-34.
Kelly, P.J., Eisman, J.A., Stuart, M.C., Pocock, N.A., Sambrook, P.N., Gwinn, T.H., Somatomedin-C, physical fitness, and bone density, “Journal of Clinical Endocrinology and Metabolism”, 1990, 70, pp. 718-723.
Kim, B., Feldman, E.L., Insulin-like growth factor I prevents mannitol-induced degradation of focal adhesion kinase and Akt, “Journal of Biological Chemistry”, 2002, 277 (30) pp. 27393-27400.
Kim, J.G., Roh, K.R., Lee, J.Y., The relationship among serum insulin-like growth factor-I, insulin-like growth factor-I gene polymorphism, and bonemineral density in postmenopausal women in Korea, “American Journal of Obstetrics and Gynecology”, 2002, 186 (3), pp. 345-350.
Klibansky, A., Billen, B.M.K., Schoenfeld, D.A., Herzog, D.B., Saxe, V.C., The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa, “Journal of Clinical Endocrinology and Metabolism”, 1995, vol. 80, pp. 898-904.
Kooijman, R., Coppens, A., Hooghe-Peters, E., Igf-I inhibits spontaneous apoptosis in human granulocytes, “Endocrinology”, 2002, 143 (4), pp. 1206-1212.
Koziris, L.P., Hickson, R.C., Chatterton, R.T., Groseth, R.T., Christe, J.M., Goldflies, D.G., Unterman, T.G., Serum levels of total and free IGF-I andIGFBP-3 are increased and maintained in long-term training, “Journal of Applied Physiology”, 1999, 86, pp. 1436-1442.
Krzysiek, J., Milewicz, T., Augustowska, K., Sztefko, K., Rys, J., Zubel, A., Pitynski, K., Jaszczynski, P., Herman, K., Basta, A., Stachura, J., Gregoraszczuk, E.L., The impact of progesterone on simultaneous, local secretion of IGFBP-3 and IGF-I [IGFBP-3/IGF-I index] by human malignant and non-malignant breast explants depends on tissue steroid receptor phenotype, “Ginekologia Polska”, 2003, 74 (9), pp. 767-774.
Kunitomi, M., Wada, J., Takahashi, K., Tsuchiyama, Y., Mimura, Y., Hida, K., Miyatake, N., Fujii, M., Kira, S., Shikata, K., Maknio, H., Relationship between reduced serum IGF-I levels and accumulation of visceral fat in Japanese men, “International Journal of Obesity Related to Metabolism Disorders”, 2002, 26 (3), pp. 361-369.
Lalous, C., Silve, C., Rosato, R., Segovia, B., Binoux, M., Interactions between insulin-like growth factor-I (IGF-I) and the system of plasminogen activators and their inhibitors in the control of IGF-binding protein-3 production and proteolysis in human osteosarcoma cells, “Endocrinology”, 1994, vol. 135, pp. 2318-2326.
Lam, S., van der Geest, R.N., Verhagen, N.A., van Nieuwenhoven, F.A., Blom, I.E., Aten, J., Goldschmeding, R., Daha, M.R., van Kooten, C., Connective tissue growth factor and igf-I are produced by human renal fibroblasts and cooperate in the induction of collagen production by high glucose, “Diabetes”, 2003, 52 (12), pp. 2975-2983.
Landin-Wilhelmsen, K., Wilhelmsen, L., Lappas, G., Rosén, T.,Lindstedt, G., Lundberg, P.-A., Bengtsson, B.A., Serum insulin-like growth factor I in a random population sample of men and women: relation to age, sex, smoking habits, coffee consumption and physical activity, blood pressure and concentrations of plasma lipids, fibrinogen, parathyroid hormone andosteocalcin, “Clinical Endorinology”, 1994, 41, pp. 351-357.
Laughlin, G.A., Barrett-Connor, E., Criqui, M.H., Kritz-Silverstein, D., The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: the Rancho Bernardo Study, “Journal of Clinical Endocrinology and Metabolism”, 2004, 89 (1), pp. 114-120.
Lewinski, A., Marcinkowska, M., Brzezianska, E., Jeziorowska, A., Wloch, J., Brzezinski, J., Expression of insulin-like growth factor I (IGF-I) gene and of genes for IGF-binding proteins 1, 2, 3, 4 (IGFBP-1-IGFBP-4) in non-neoplastic human thyroid cells and in certain human thyroid cancers. Effect of exogenous IGF-I on this expression, “Endocrinology Research”, 2004, 30 (1), pp. 47-59.
Liu, E., Law, H.K., Lau, Y.L., Insulin-like growth factor I promotes maturation and inhibits apoptosis of immature cord blood monocyte-derived dendritic cells through MEK and PI 3-kinase pathways, “Pediatric Research”, 2003, 54 (6), pp. 919-925.
Lu, Y., Zi, X., Zhao, Y., Pollak, M., Overexpression of ErbB2 receptor inhibits IGF-I-induced Shc-MAPK signaling pathway in breast cancer cells, “Biochemical and Biophysical Research Communications”, 2004, 313 (3), pp. 709-715.
Lukanova, A., Lundin, E., Toniolo, P., Micheli, A., Akhmedkhanov, A., Rinaldi, S., Muti, P., Lenner, P., Biessy, C., Krogh, V., Zeleniuch-Jacquotte, A., Berrino, F., Hallmans, G., Riboli, E., Kaaks, R., Circulating levels of insulin-like growth factor-I and risk of ovarian cancer, “International Journal of Cancer”, 2002, 101 (6), pp. 549-554.
Machida, S., Spangenburg, E.E., Booth, F.W., Forkhead transcription factor FoxO1 transduces insulin-like growth factor's signal to p27Kip1 in primary skeletal muscle satellite cells, “Journal of Cellular Physiology”, 2003, 196 (3), pp. 523-531.
Manetta, J., Brun, J.F., Fedou, C., Maimoun, L., Prefaut, C., Mercier, J., Serum levels of insulin-like growth factor-I (IGF-I), and IGF-binding proteins-1 and -3 in middle-aged and young athletes versus sedentary men: relationship with glucose disposal, “Metabolism”, 2003, 52 (7), pp. 821-826.
Manetta, J., Brun, J.F., Maimoun, L., Callis, A., Préfaut, C., Mercier, J., Effect of training on GH/IGF-1 axis during exercise in middle-aged men:relationship to glucose homeostasis, “American Journal of Physiology and Endocrinology Metabolism”, 2002, 283, pp. E929-E936.
Musaro, A., McCullagh, K. J. A., Naya, F. J., Olson, E. N., Rosenthal, N., IGF-1 induces skeletal myocyte hypertrophy through calcineurin inassociation with GATA-2 and NF-ATc1, “Nature”, 1999,400, pp. 581-585.
Nemet, D., Connolly, P.H., Pontello-Pescatello, A.M., Rose-Gottron, C., Larson, J.K., Galassetti, P., Cooper, D.M., Negative energy balance plays a major role in the IGF-I response to exercise training, “Journal of Applied Physiology”, 2004, 96 (1), pp. 276-282.
Niall, H.D., Hogan, M.L., Sauer, R., Rosenblum, I. Y., Greenwood, F.C., Sequence of pituitary and placental lactogenic and growth hormones: evolution from a primordial peptide by gene reduplication, “Proceedings of the National Academy of Sciences”, 1971, 68, pp. 866-869.
Nindl, B.C., Castellani, J.W., Young, A.J., Patton, J.F., Khosravi, M.J., Diamandi, A., Montain, S.J., Differential responses of IGF-I molecularcomplexes to military operational field training, “Journal of Applied Physiology”, 2003, 95 (3), pp. 1083-1089.
Obrepalska-Steplowska, A., Kedzia, A., Gozdzicka-Jozefiak, A., Jakubowicz, M., Korman, E., Analysis of the human growth hormone receptor and IGF-I coding sequences in children with growth disorders, “Journal of Pediatrics Endocrinology and Metabolism”, 2003, 16 (6), pp. 819-825.
Obrepalska-Steplowska, A., Kedzia, A., Trojan, J., Gozdzicka-Jozefiak, A., Analysis of coding and promoter sequences of the IGF-I gene in children with growth disorders presenting with normal level of growth hormone, “Journal of Pediatric Endocrinology and Metabolism”, 2003, 16 (9), pp. 1267-1275.
Oh, J.S., Kucab, J.E., Bushel, P.R., Martin, K., Bennett, L., Collins, J., DiAugustine, R.P., Barrett, J.C., Afshari, C.A., Dunn, S.E., Insulin-like growth factor-1 inscribes a gene expression profile for angiogenic factors and cancer progression in breast epithelial cells, “Neoplasia”, 2002, 4 (3), pp. 204-217.
Olchovsky, D., Shimon, I., Goldberg, I., Shulimzon, T., Lubetsky, A., Yellin, A., Pariente, C., Karasik, A., Kanety, H., Elevated insulin-like growth factor-1 and insulin-like growth factor binding protein-2 in malignant pleural effusion, “Acta Oncologica”, 2002, 41 (2), pp. 182-187.
Poehlman, E.T., Copeland, K.C., Influence of physical activity on insulin-like growth factor-I in healthy younger and older men, “Journal of Clinical Endocrinology and Metabolism”, 1990, 71, pp. 1468-1473.
Popken, G.J., Hodge, R.D., Ye, P., Zhang, J., Ng, W., O'Kusky, J.R., D'Ercole, A.J., In vivo effects of insulin-like growth factor-I (IGF-I) on prenatal and early postnatal development of the central nervous system, “European Journal of Neurosciences”, 2004, 19 (8), pp. 2056-2068.
Psilander, N., Damsgaard, R., Pilegaard, H., Resistance exercise alters MRF and IGF-I mRNA content in human skeletal muscle, “Journal of Applied Physiology”, 2003, 95 (3), pp. 1038--1044.
Raile, K., Hille, R., Laue, S., Schulz, A., Pfeifer, G., Horn, F., Kiess, W., Insulin-like growth factor I (IGF-I) stimulates proliferation but also increasescaspase-3 activity, Annexin-V binding, and DNA-fragmentation in human MG63 osteosarcoma cells: co-activation of pro- and anti-apoptotic pathways by IGF-I, “Hormone and Metabolic Research”, 2003, 35 (11-12), pp. 786--793.
Rajaram, S., Baylink, D.J., Mohan, S., Insulin-like growth factor-bindingproteins in serum and other biological fluids: regulation and functions, “Endocrinology Review”, 1997, 18, pp. 801-831.
Rasmussen, S.K., Lautier, C., Hansen, L., Echwald, S. M., Hansen, T., Ekstrom, C.T., Urhammer, S.A., Borch-Johnsen, K., Grigorescu, F., Smith, R.J., Pedersen, O., Studies of the variability of the genes encoding the insulin-like growth factor I receptor and its ligand in relation to type 2 diabetes mellitus. “Journal of Clinical Endocrinology and Metabolism”, 2000, 85, pp. 1606-1610.
Rietveld, I., Janssen, J.A., Hofman, A., Pols, H.A., van Duijn, C.M., Lamberts, S.W., A polymorphism in the IGF-I gene influences the age-related decline in circulating total IGF-I levels, “European Journal of Endocrinology”, 2003, 148 (2), pp. 171-175.
Rinderknecht, E., Humbel, R.E., The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin, “Journal of Biological Chemistry” 1978, 253, pp. 2769-2776.
Rivadeneira, F., Houwing-Duistermaat, J.J., Vaessen, N., Vergeer-Drop, J.M., Hofman, A., Pols, H.A., Van Duijn, C.M., Uitterlinden, AG., Density in the elderly: the Rotterdam Study, “Journal of Clinical Endocrinology and Metabolism”, 2003, 88 (8), pp. 3878-3884.
Rosendal, L., Langberg, H., Flyvbjerg, A., Frystyk, J., Ørskov, H., Kjaer, M., Physical capacity influences the response of insulin-like growth factor and itsbinding proteins to training, “Journal of Applied Physiology”, 2002, 93, pp. 1669-1675.
Rotwein, P., Two insulin-like growth factor I messenger RNAs are expressed in human liver, “Proceedings of the National Academy of Sciences”, 1986, 83, pp. 77-81.
Rubin, J., Chung, L.W., Fan, X., Zhu, L., Murphy, T.C., Nanes, M.S., Rosen, C.J., Prostate carcinoma cells that have resided in bone have an upregulated IGF-I axis, “Prostate”, 2004, 58 (1), pp. 41-49.
Sadowski, T., Dietrich, S., Koschinsky, F., Sedlacek, R., Matrix metalloproteinase 19 regulates insulin-like growth factor-mediated proliferation, migration, and adhesion in human keratinocytes through proteolysis of insulin-like growth factor binding protein-3, “Molecular Biology of the Cell”, 2003, 14 (11), pp. 4569-4580.
Saeki, H., Hamada, M., Hiwada, K., Circulating levels of insulin-like growth factor-1 and its binding proteins in patients with hypertrophic cardiomyopathy, “Circulation Journal”, 2002, 66 (7), pp. 639-644.
Sakai, K., Lowman, H.B., Clemmons, D.R., Increases in free, unbound insulin-like growth factor I enhance insulin responsiveness in human hepatoma G2 cells in culture, “Journal of Biological Chemistry”, 2002, 277 (16), pp. 13620-13627.
Scavo, L.M., Karas, M., Murray, M., Leroith, D., Insulin-like growth factor-I stimulates both cell growth and lipogenesis during differentiation of human mesenchymal stem cells into adipocytes, “Journal of Clinical Endocrinology and Metabolism”, 2004, 89 (7), pp. 3543-3553.
Schut, A.F., Janssen,, J.A., Deinum, J., Vergeer, J.M., Hofman, A., Lamberts, S.W., Oostra, B.A., Pols, H.A., Witteman, J.C., van Duijn, C.M., Polymorphism in the promoter region of the insulin-like growth factor I gene is related to carotid intima-media thickness and aortic pulse wave velocity in subjects with hypertension, “Stroke”, 2003, 34 (7), pp. 1623-1627.
Schwarz, A.J., Brasel, J.A., Hintz, R.L., Mohan, S., Cooper, D.M., Acute effect of brief low and high-intensity exercise on circulating insulin-like growth factor (IGF) I, II, and IGF-binding protein-3 and its proteolysis in young healthymen, “Journal of Clinical Endocrinology and Metabolism”, 1996, 81, pp. 3492-3497.
Semsarian, C., Wu, M.-J., Ju, Y.-K., Marciniec, T., Yeoh, T., Allen, D. G., Harvey, R. P., Graham, R. M., Skeletal muscle hypertrophy is mediated bya Ca(2+)-dependent calcineurin signalling pathway, “Nature”, 1999,400, pp. 576-581.
Shi, R., Yu, H., McLarty, J., Glass, J., IGF-I and breast cancer: a meta-analysis, “International Journal of Cancer”, 2004, 111 (3), pp. 418-423.
Sjogren, K., Liu, J.-L., Blad, K., Skrtic, S., Vidal, O., Wallenius, V., LeRoith, D., Tornell, J., Isaksson, O. G. P., Jansson, J.-O., Ohlsson, C., Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-Iin blood but is not required for postnatal body growth in mice, “Proceedings of National Academy of Sciences USA”, 1999,96, pp. 7088-7092.
Skalkidou, A., Petridou, E., Papathoma, E., Salvanos, H., Kedikoglou, S., Chrousos, G., Trichopoulos, D., Determinants and consequences of major insulin-like growth factor components among full-term healthy neonates, “Cancer Epidemiology Biomarkers and Prevention”, 2003, 12 (9), pp. 860-865.
Smith, P.J., Spurrell, E.L., Coakley, J., Hinds, C.J., Ross, R.J., Krainer, A.R., Chew, S.L., An exonic splicing enhancer in human IGF-I pre-mRNA mediates recognition of alternative exon 5 by the serine-arginine proteinsplicing factor-2/alternative splicing factor, “Endocrinology”, 2002, 143, pp. 146-154.
Sneppen, S.B., Lange, M., Pedersen, L.M., Kristensen, L., LO, Main, K.M., Juul, A., Skakkebaek, N.E., Feldt-Rasmussen, U., Total and free insulin-like growth factor I, insulin-like growth factor binding protein 3 and acid-labile subunit reflect clinical activity in acromegaly, “Growth Hormone and IGF Research”, 2001, 11 (6), pp. 384-391.
Sorensen, H., Whittaker, L., Hinrichsen, J., Groth, A., Whittaker, J., Mapping of the insulin-like growth factor II binding site of the Type I insulin-like growth factor receptor by alanine scanning mutagenesis, “FEBS Letters”, 2004, 565 (1-3), pp. 19-22.
Spangenburg, E.E., Bowles, D.K., Booth, F.W., Insulin-like growth factor-induced transcriptional activity of the skeletal alpha-actin gene is regulated by signaling mechanisms linked to voltage-gated calcium channels during myoblast differentiation, “Endocrinology”, 2004, 145 (4), pp. 2054-2063.
Strammiello, R., Benini, S., Manara, M.C., Perdichizzi, S., Serra, M., Spisni, E., Picci, P., Scotlandi, K., Impact of IGF-I/IGF-IR circuit on the angiogenetic properties of Ewing's sarcoma cells, “Hormone and Metabolic Research”, 2003, 35 (11-12), pp. 675-684.
Sweeney H.L., Genowy doping, “Świat Nauki”, 2004, 8 (156), pp. 33-39.
Umayahara, Y., Kajimoto, Y., Fujitani, Y., Gorogawa, S., Yasuda, T., Kuroda, A., Ohtoshi, K., Yoshida, S., Kawamori, D., Yamasaki, Y., Hori, M., Protein kinase C-dependent, CCAAT/enhancer-binding protein beta-mediated expression of insulin-like growth factor I gene, “Journal of Biological Chemistry”, 2002, 277 (18), pp. 15261-15270.
Vaessen, N., Janssen, J.A., Heutink, P., Hofman, A., Lamberts, S.W.J., Oostra, B.A., Pols, H.A.P., van Duijn, C. M., Association between genetic variation in the gene for insulin-like growth factor-1 and low birthweight. “Lancet”, 2002, 359, pp. 1036-1037.
Walsh, S., Jefferiss, C.M., Stewart, K., Beresford, J.N., IGF-I does not affect the proliferation or early osteogenic differentiation of human marrow stromal cells, “Bone”, 2003, 33 (1), pp. 80-89.
Watanabe, T., Yamamoto, H., Idei, T., Iguchi, T., Katagiri, T., Influence of insulin-like growth factor-1 and hepatocyte growth factor on carotid atherosclerosis and cognitive function in the elderly, “Dementia and Geriatric Cognitive Disorders”, 2004,18 (1), pp. 67-74.
Waters, D.L., Yau, C.L., Montoya, G.D., Baumgartner, R.N., Serum Sex Hormones, IGF-1, and IGFBP3 Exert a Sexually Dimorphic Effect on Lean Body Mass in Aging, “Journal of Gerontology series A: Biological Sciences and Medical Sciences”, 2003, 58 (7), pp. 648-652.
Welle, S., Bhatt, K., Shah, B., Thornton, C., Insulin-like growth factor-1 and myostatin mRNA expression in muscle: comparison between 62-77 and 21-31 yr old men, “Experimen-tal Gerontology”, 2002, 37 (6), pp. 833-839.
Winn, N., Paul, A., Musaro, A., Rosenthal, N., Insulin-like growth factor isoforms in skeletal muscle aging, regeneration, and disease, “Cold Spring Harbour Symposium on Quantitative Biology”, 2002, 67, pp. 507-518.
Woods, K. A., Camacho-Hubner, C., Savage, M. O., Clark, A. J. L., Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. “New England Journal of Medicine”, 1996,335, pp. 1363-1367.
Yakar, S., Liu, J.-L., Stannard, B., Butler, A., Accili, D., Sauer, B., LeRoith, D., Normal growth and development in the absence of hepaticinsulin-like growth factor I, “Proceedings of National Academy of Sciences”, 1999,96, pp. 7324-7329.
Young, S.C.J., Underwood, L.E., Celniker, A., Clemmons, D.R., Effects of recombinant insulin-like growth factor-I (IGF-I) and growth hormone on serum IGF-binding proteins in calorically restricted adults, “Journal of Clinical Endocrinology and Metabolism”, 1992, 75, pp. 603-608.
Zanconato, S., Moromisato, D.Y., Moromisato, M.Y., Woods, J., Brasel, J.A., Leroith, D., Roberts, C.T. Jr., Cooper, D.M., Effect of training and growth hormone suppression on insulin-like growth factor I mRNA in young rats, “Journal of Applied Physiology”, 1994, 76, pp. 2204-2209.
Zhu, J., Kahn, C. R., Analysis of a peptide hormone-receptor interaction in the yeast two-hybrid system, “Proceedings of National Academy of Sciences”, 1997,94, pp. 13063-13068.